 Tamayakin’ initiative
Tamayakin’ initiative
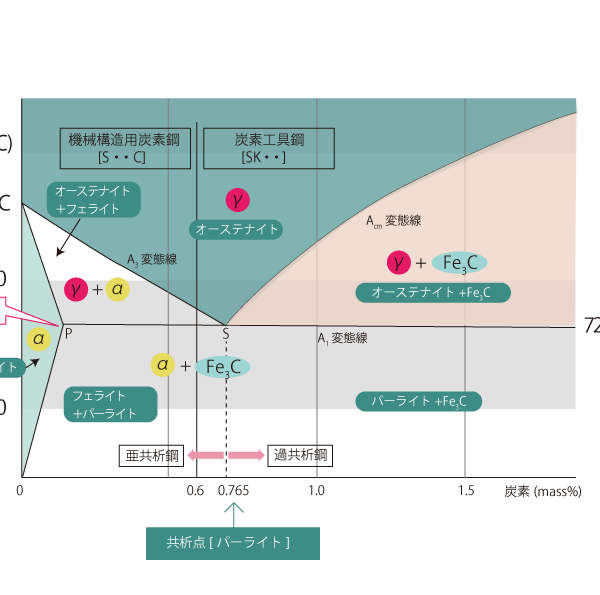
Metallurgical structure of carbon steel at equilibrium

 Return to list
Return to list
Overview.
The 0.765% carbon content point is called the eutectic point, and carbon steel containing that amount of carbon is called eutectoid steel. The metallurgical structure at this point is commonly called pearlite.
01 Equilibrium state diagram and metallography
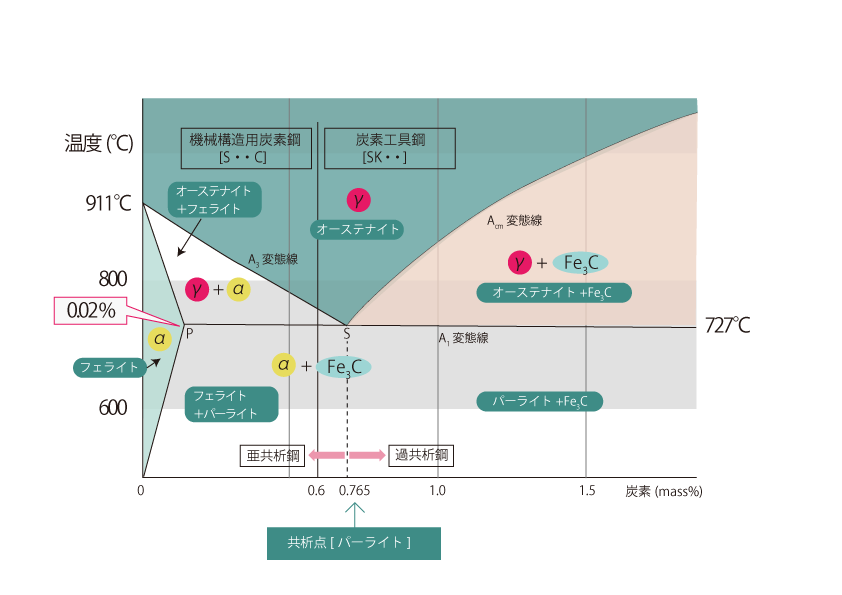
In this equilibrium state diagram, carbon steels with carbon content less than 0.6% are carbon steels for machine structural use, and those with carbon content greater than 0.6% are carbon tool steels. The 0.765% carbon content point is called the eutectic point, and carbon steels with this carbon content are called eutectoid steels, those with less carbon content than this point are called sub-eutectoid steels, and those with more carbon content are called hyper-eutectoid steels.
02 Metallographic structure of sub-eutectoid steel
The metallurgical structure at higher temperatures than the A3 line is austenite. As it cools, ferrite precipitates as it passes through the A3 line. This is called Initially precipitated ferrite and is called Furthermore, ferrite and cementite ( Fe3C ) precipitate simultaneously from austenite as it passes the A1 line. This transformation from one phase to two phases simultaneously is called eutectic transformation, and the metallographic structure obtained by this eutectic transformation is called pearlite (ferrite and Fe3C are arranged in layers).
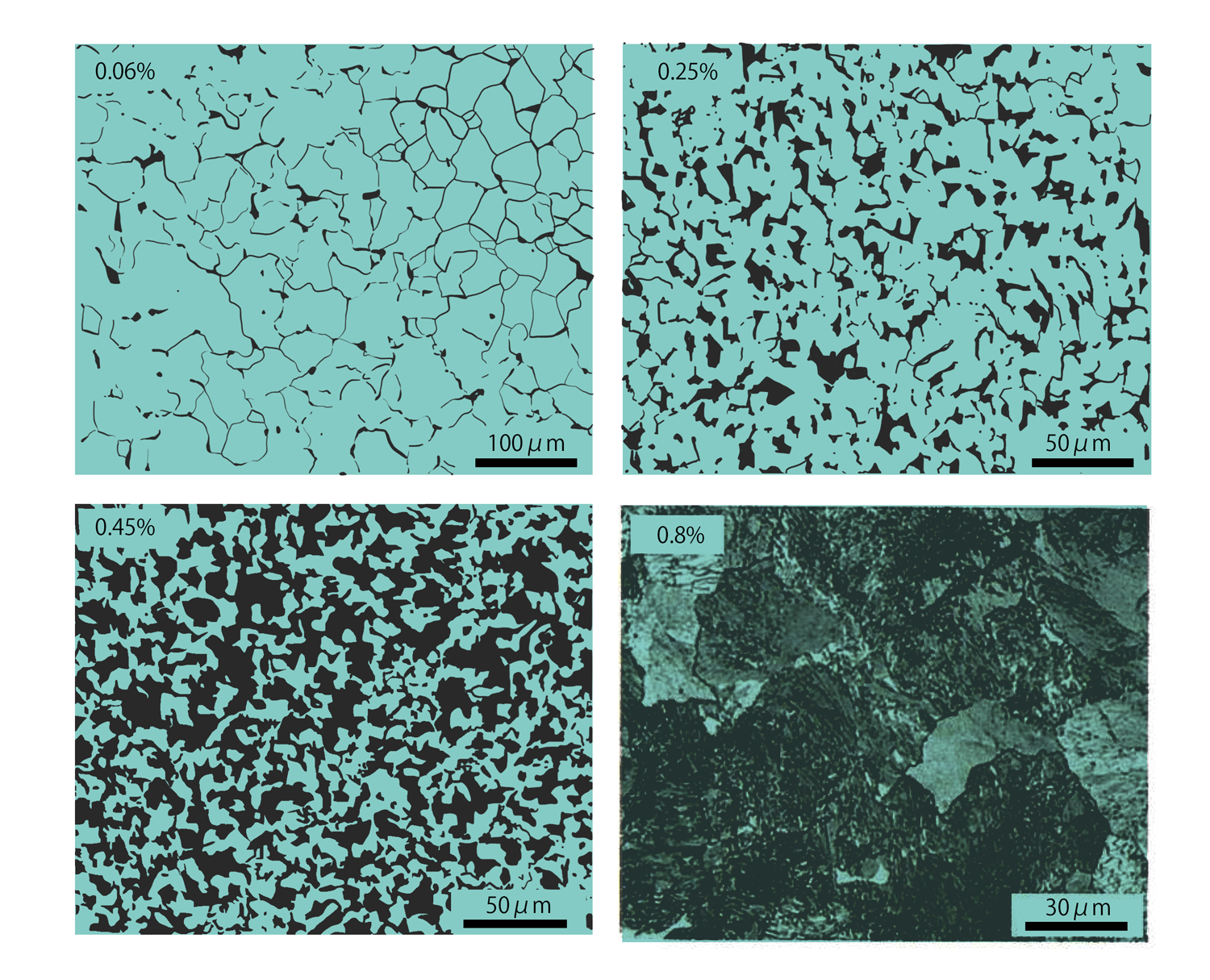
The microscopic microstructures of various carbon steels with different carbon contents are shown above, which exhibit a mixed structure of pearlite and primary ferrite in equilibrium below the A1 transformation point. Furthermore, the amount of pearlite increases with carbon content, and at 0.8% carbon content, which is the eutectic point, all of the carbon content is pearlite.
03 Metallographic structure of percolloidal steel
A characteristic feature of hypercoagulating steel is the presence of the Acm line, at which temperature cementite ( Fe3C ) precipitates from austenite, which is called primary cementite (Fe3C). The amount of cementite increases as one gets closer to the A1 line, and since the A1 line undergoes pearlitic transformation, the metallurgical structure at room temperature is a mixture of pearlite and precipitated Fe3C.



