Tamayakin’ initiative
Tamayakin’ initiative
Temperature and structure of pure iron

 Return to list
Return to list
Overview.
As pure iron in steel rises in temperature from room temperature, it undergoes two structural and dimensional changes before reaching its melting point.
01 Structural changes with increasing temperature
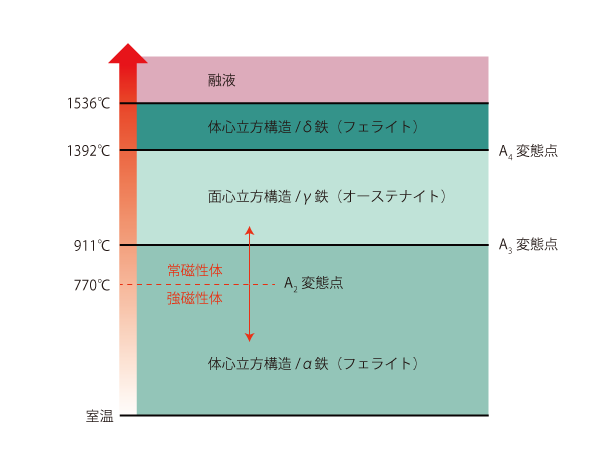
Relationship between temperature, structure and metallurgy of pure iron
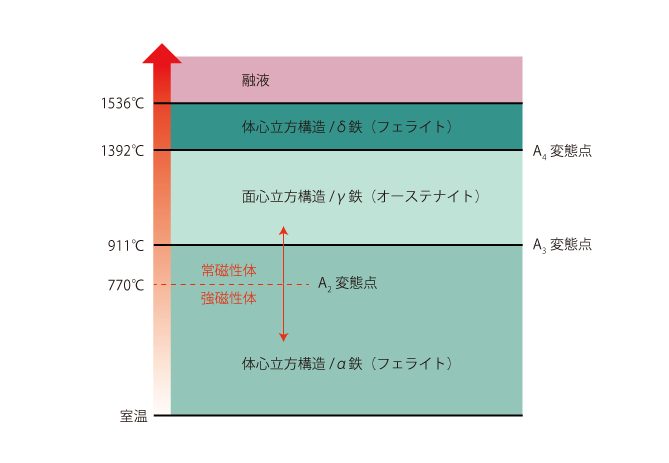
The crystal structure at room temperature is body-centered cubic structure and the metallurgical structure is alpha iron (ferrite).
As the temperature rises to 770°C, it changes from ferromagnetic to paramagnetic at the A2 transformation point. In other words, iron will stick to a magnet until it reaches 770°C, but will not stick to a magnet at temperatures higher than 770°C.
At the A3 transformation point (911°C), the crystal structure changes to a face-centered cubic structure and the metallurgical structure changes to γ-iron (austenite). At the A4 transformation point (1392°C), the crystal structure changes to a body-centered cubic structure and the metallurgical structure changes to delta iron (delta ferrite).
02 Body-centered cubic and face-centered cubic structures
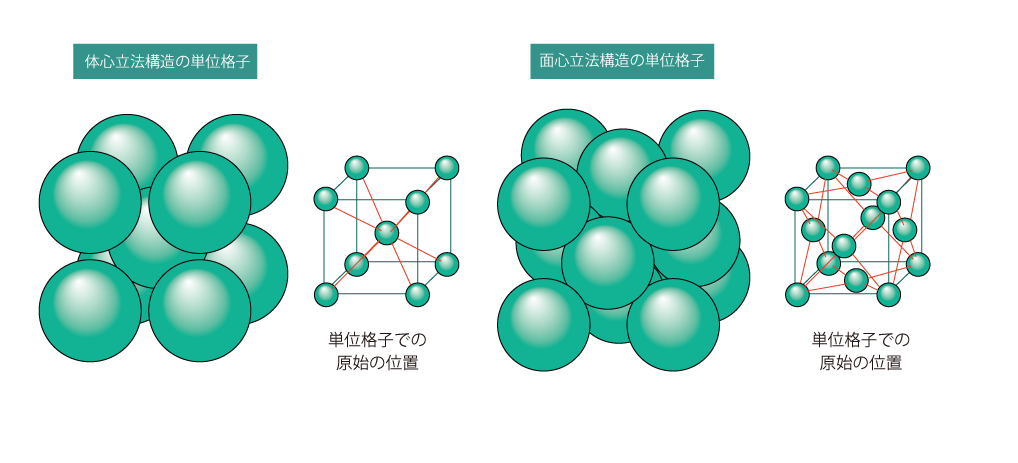
Body-centered cubic structure is a structure in which atoms are located in the center of the lattice. Body-centered cubic structure is called bcc (body-centered-cubic) structure for short. A face-centered cubic structure is a structure in which atoms are located on the surface of the lecturer. The face-centered cubic structure is called fcc (face-centered-cubic) structure for short. fcc iron is softer and more changeable than bcc iron.
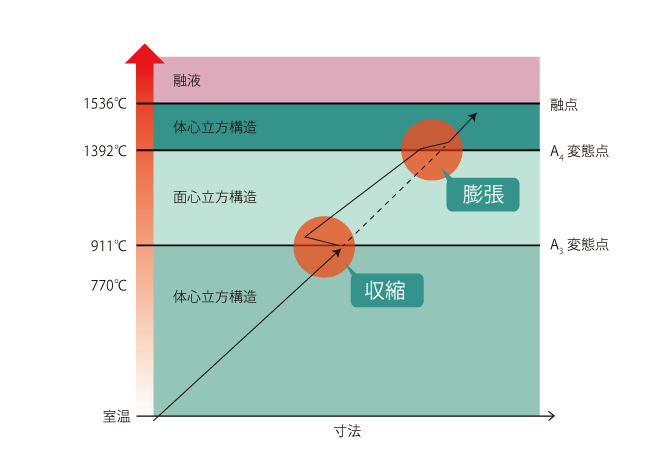
03 Dimensional change with temperature increase
Comparing the dimensions of a body-centered and face-centered structure, the face-centered structure has smaller dimensions.
Comparing body-centered and face-centered structures with the same number of atoms, it is clear that the volume of the face-centered structure is smaller because the body-centered structure has two lattice units and the face-centered structure has four lattice units.
Therefore, at the A3 transformation point, iron changes from body-centered to face-centered cubic structure, so its dimensions contract, and at the A4 transformation point, it changes from face-centered to body-centered cubic structure, so its dimensions expand.