Tamayakin’ initiative
Tamayakin’ initiative

 Return to list
Return to list
Overview.
Iron ore is turned into pig iron using a blast furnace. Impurities are removed from the pig iron to produce steel.
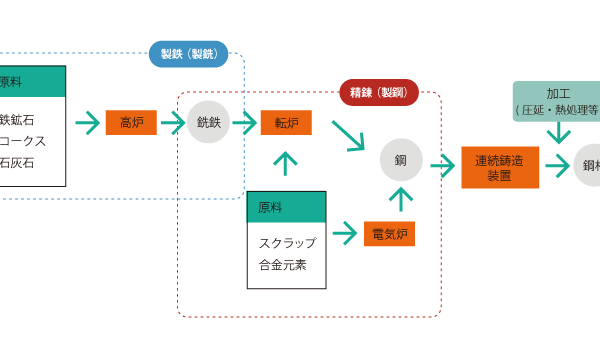
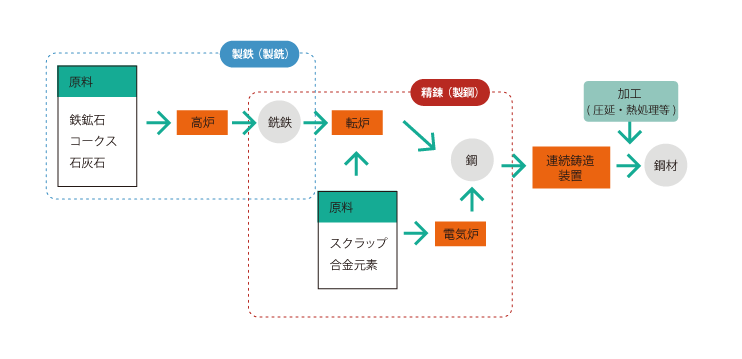
01 Steel Manufacturing Process
Iron ore, coke, and limestone are used as raw materials, and blast furnaces are used to produce pig iron from iron ore, coke, limestone, etc. using a blast furnace. pig iron making The process of making pig iron is called pig iron making.
The next step after iron making is refining process.
In refining, impurities such as phosphorus (P), sulfur (S), and silicon (Si) are removed from the pig iron to produce steel.
At this point, a converter furnace or electric furnace is used.
The steel is then passed through continuous casting equipment to complete the steel product.
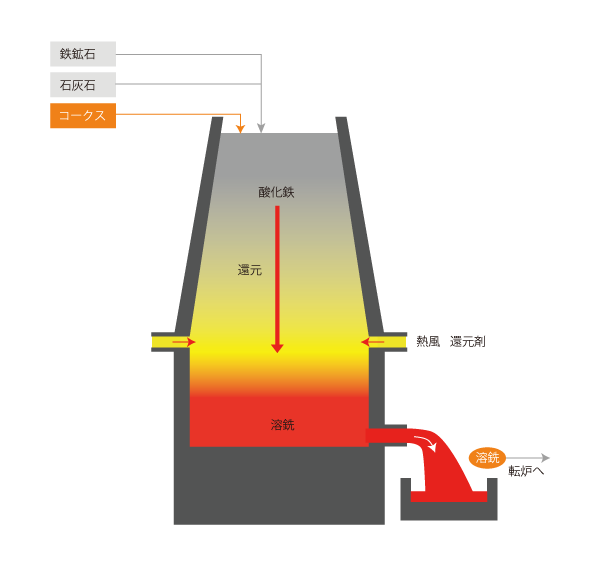
02 Iron making by blast furnace
When iron ore, limestone, and coke are placed in the blast furnace, the iron oxide descends. The coke turns into reducing gas, which is raised by hot air. The reducing gas reduces the iron oxide and accumulates below as hot metal.
The accumulated hot metal is transferred to the converter as pig iron.
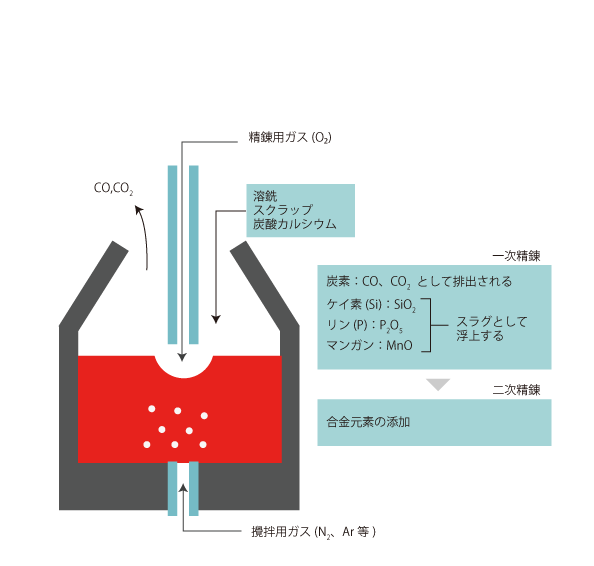
03 Refining by converter furnace
Hot metal produced in a blast furnace contains too much carbon, silicon, phosphorus, and manganese.
To remove those impurities, they are oxidized and removed as oxides.
Alloying elements are then added to adjust the composition.
The arc furnace is a typical example of an electric furnace. The raw material is steel scrap. Alloying elements are added to produce steel.